
Patients were given 4 cycles of treatment followed by pembrolizumab or placebo was with maintenance pemetrexed.
#Keynote 189 overall survival update plus#
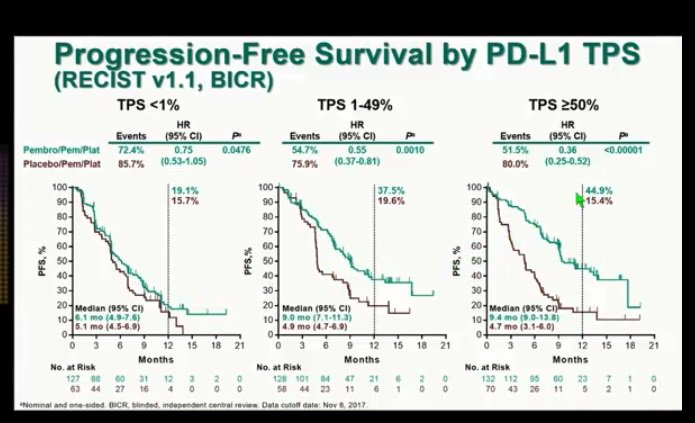
Patients were randomized 2:1 in the phase III trial, receiving either pembrolizumab (n = 410) or placebo (n = 206) plus pemetrexed and carboplatin or cisplatin. During the 2019 ASCO Annual Meeting, updates to the study were presented, showing a longer follow-up and progression-free survival 2 (PFS2) findings.
#Keynote 189 overall survival update trial#
All rights reserved.The phase III KEYNOTE-189 trial led to the FDA approval of the combination of pembrolizumab (Keytruda) plus chemotherapy for the treatment of patients with metastatic nonsquamous nonsmall cell lung cancer (NSCLC) without EGFRor ALKalterations. This regimen is a standard-of-care treatment option for treatment-naive patients with advanced NSCLC, including patients with stable brain metastases.īrain metastases Chemotherapy Non‒small-cell lung cancer PD-L1 Pembrolizumab.Ĭopyright © 2021 Merck Sharp & Dohme Corp.
With or without brain metastasis, pembrolizumab plus platinum-based histology-specific chemotherapy improved clinical outcomes versus chemotherapy alone across all programmed death ligand 1 subgroups, including patients with programmed death ligand 1 tumor proportion score less than 1% and had a manageable safety profile in patients with advanced NSCLC. Incidences of treatment-related adverse events with pembrolizumab plus chemotherapy versus chemotherapy were 88.2% versus 82.8% among patients with brain metastases and 94.5% versus 90.6% in those without. Objective response rates were higher and duration of response longer with pembrolizumab plus chemotherapy versus chemotherapy regardless of brain metastasis status. In patients with brain metastases, median overall survival was 18.8 months with pembrolizumab plus chemotherapy and 7.6 months with chemotherapy, and median progression-free survival was 6.9 months and 4.1 months, respectively. Hazard ratios (pembrolizumab + chemotherapy/chemotherapy) were similar for patients with and without brain metastases for overall survival (0.48 and 0.63, respectively) and progression-free survival (0.44 and 0.55, respectively). Median (range) durations of follow-up at data cutoff were 10.9 (0.1‒35.1) and 11.0 (0.1‒34.9) months, respectively. Patients with known untreated asymptomatic brain metastases required regular imaging of the brain.Ī total of 1298 patients were included, 171 with and 1127 without baseline brain metastases. Patients with previously treated brain metastases were clinically stable for 2 or more weeks (≥4 wk in KEYNOTE-021 cohort G), had no evidence of new or enlarging brain metastases, and had no steroid use at least 3 days before dosing. All studies permitted enrollment of patients with previously treated or untreated (KEYNOTE-189 and KEYNOTE-407 only) stable brain metastases. Patients were assigned to platinum-doublet chemotherapy with or without the addition of 35 cycles of pembrolizumab 200 mg every 3 weeks. We pooled data for patients with advanced NSCLC in KEYNOTE-021 cohort G (nonsquamous), KEYNOTE-189 (nonsquamous), and KEYNOTE-407 (squamous). This exploratory analysis retrospectively evaluated outcomes in patients with advanced NSCLC to determine whether baseline brain metastases influenced the efficacy of first-line pembrolizumab plus chemotherapy versus chemotherapy alone.


 0 kommentar(er)
0 kommentar(er)
